HBN وCBN وWBN: تحليل مقارن لمتعدد أشكال نيتريد البورون نيتريد
1 Introduction
In advanced materials science, boron nitride (BN) is a significant material due to its unique combination of properties. This compound, composed of the light elements boron and nitrogen, forms several polymorphs with distinct atomic arrangements, leading to markedly different physical and chemical characteristics. Among these, hexagonal boron nitride (HBN), cubic boron nitride (CBN), and wurtzite boron nitride (WBN) represent the most technologically relevant forms.
Similar to how carbon atoms arrange to form graphite and diamond, boron nitride polymorphs exhibit significant differences in properties such as hardness, thermal conductivity, and electrical insulation. HBN, often termed "white graphene", offers excellent lubricity and high-temperature stability. CBN, second only to diamond in hardness, is crucial for super-hard machining applications. WBN, a more recent addition, shows promise for semiconductor and extreme-environment applications. This article examines the crystal structures, synthesis methods, key properties, and applications of these three BN polymorphs. By comparing them, we explore the fundamental materials science principle that structure determines properties, providing a basis for material selection and design.
2 Comparison of The Basic Properties of Three Boron Nitride Materials
2.1 Crystal Structure Analysis
The diversity of boron nitride materials is first reflected in the fundamental differences in their atomic arrangements. These structural differences directly determine the basic properties of the materials:
HBN (hexagonal boron nitride): It has a layered hexagonal crystal structure (space group P6₃/mmc), with boron and nitrogen atoms in each layer connected by strong sp^2 hybridized covalent bonds, forming hexagonal rings similar to a honeycomb structure. Layers are bonded via van der Waals forces, and this weak interaction allows for easy interlayer sliding. The lattice parameters of HBN are typically a = 2.504 Å and c = 6.656 Å, with an interlayer spacing (0.333 nm) approximately equal to graphite's 0.335 nm, which is attributed to the polar nature of the B-N bonds.
CBN (cubic boron nitride): It adopts a sphalerite-type structure (space group F-43m), where each boron atom is connected to four nitrogen atoms via strong sp^3 hybridized bonds, forming a three-dimensional tetrahedral network. This dense structure makes it an ultra-hard material with hardness second only to diamond, with a lattice constant of approximately 3.615 Å. Unlike diamond, the CBN structure contains a certain amount of ionic bonding components (B+ and N-), approximately 22%, which affects its chemical stability.
WBN (wurtzite boron nitride): It has a wurtzite-type hexagonal structure (space group P6₃mc), also composed of sp^3 hybridized bonds, but the atomic stacking order differs from that of CBN (ABAB vs. ABCABC). This structure makes it a metastable phase with lattice parameters of a = 2.55 Å and c = 4.21 Å. WBN can be considered an intermediate state between HBN and CBN, combining some layered characteristics with three-dimensional bonding features.
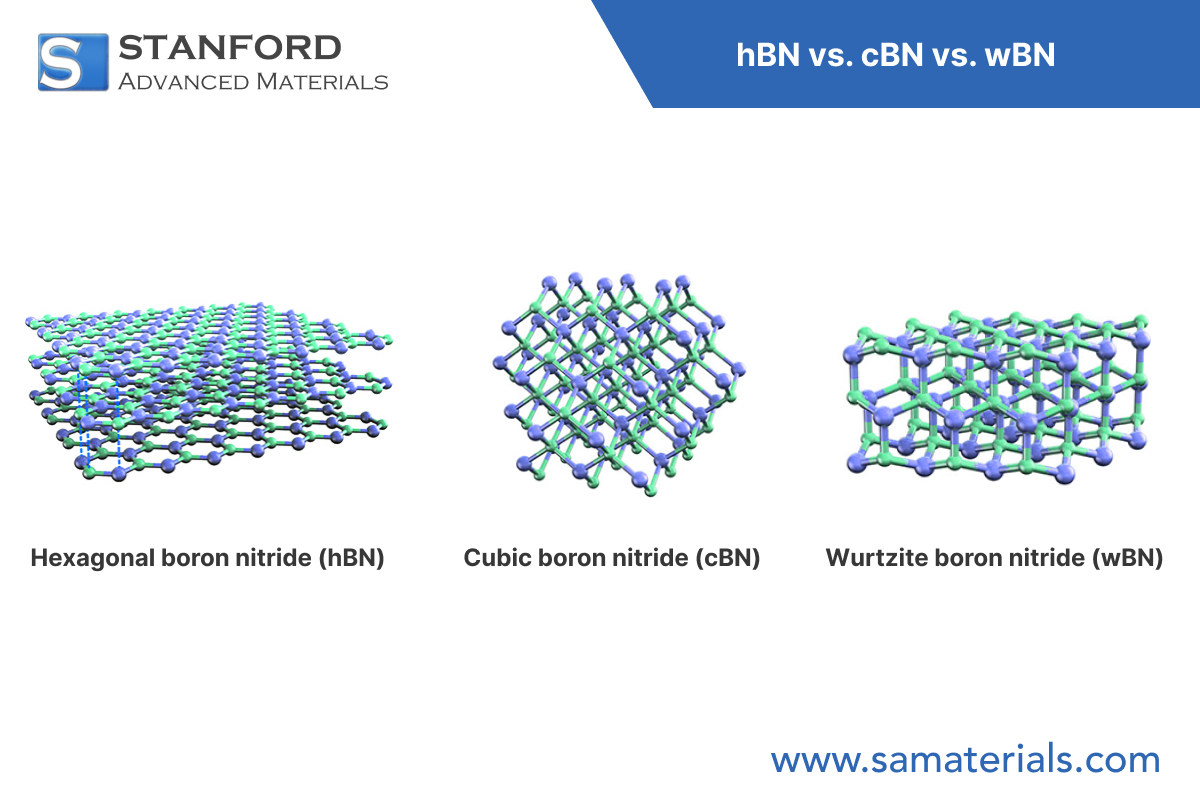
Fig. 1 Structures of Different Types of BN
2.2 Comparison of Physical and Chemical Properties
The table below summarizes the key physical and chemical properties of three boron nitride materials, which are directly derived from their crystal structure differences:
Table 1 Physical Properties of HBN, CBN, and WBN
|
Properties |
HBN |
CBN |
WBN |
|
Density (g/cm3) |
2.27-2.30 |
3.48-3.49 |
~3.49 |
|
Mohs Hardness |
1-2 |
9-9.5 |
~9.0 |
|
Thermal Conductivity (W/mK) |
∥c axis: 20-30 ⊥c axis: 2-5 |
13-20 |
15-18 |
|
Bandgap (eV) |
5.0-6.0 (indirect) |
6.1-6.4 (indirect) |
~5.8 (direct) |
|
Thermal Stability(℃) |
<900 (in air) Up to 2000 (in vacuum) |
<1400 (inert atmosphere) |
<1200 |
|
Chemical Inertness |
Resistance to molten metal erosion |
Refractory metals |
Similar to CBN but less researched |
2.3 Comparative Analysis of Superhard Material Properties
Hardness Mechanism Differences: The high hardness of CBN and WBN stems from their fully sp3-bonded three-dimensional network structure, where the strength and density of covalent bonds determine their resistance to deformation. In contrast, HBN's layered structure results in extremely low hardness, making it suitable for use as a solid lubricant.
Fracture toughness performance: CBN exhibits superior fracture toughness compared to diamond when machining iron-based alloys. This is because it does not react chemically with iron at high temperatures, avoiding the diffusion wear issues encountered with diamond tools when processing steel.
Thermal stability limits: CBN remains stable at temperatures between 1300-1400°C, while diamond begins to graphitize above 800°C. WBN's thermal stability lies between HBN and CBN, but in an oxidizing environment, all boron nitrides gradually oxidize above 800°C.
3 HBN: Structure and Applications
3.1 Structural Characteristics and Preparation Process
The hexagonal boron nitride (HBN) layered structure confers it with a unique combination of properties. Within each layer, boron and nitrogen atoms form nearly planar hexagonal rings. Due to the polarity of the B-N bonds (nitrogen atoms are slightly negatively charged, while boron atoms are slightly positively charged), electrostatic interactions exist between adjacent rings, resulting in bond strengths within HBN layers that are higher than those in graphite. This structural feature can be observed via transmission electron microscopy (TEM), revealing the layered stacking and hexagonal diffraction patterns.
HBN is mainly prepared industrially through the following methods:
Chemical vapor deposition (CVD): In a high-temperature reaction chamber (1000–1800°C), boron-containing precursors (such as B2H6 and BBr3) react with ammonia to deposit high-quality HBN films on the substrate. By controlling the nucleation density and growth temperature, HBN films of different thicknesses and grain sizes can be obtained.
High-Temperature High-Pressure Method (HTHP): Mixing boric acid with nitrogen-containing compounds (e.g., urea) and reacting them at 5 GPa and 1500°C yields bulk HBN. This method produces highly crystalline products but is costly.
Borax-urea method: Mixing borax (Na2B4O7) with urea (CO(NH2)2) and reacting at 900–1000°C in an ammonia gas stream is an economical and efficient method, but the product may contain impurities.
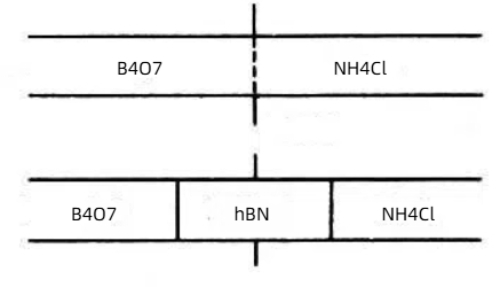
Fig. 2 Synthesis of Hexagonal Boron Nitride by Solid-Phase Reaction Method
3.2 Core Performance Advantages and Application Scenarios
The performance advantages of HBN are primarily reflected in its high-temperature stability and anisotropic properties:
Solid lubrication field: HBN has a Mohs hardness of only 1-2 and low interlayer shear strength, making it the preferred choice for high-temperature solid lubricants. It maintains a stable coefficient of friction (0.2-0.4) from room temperature to 1000°C, making it particularly suitable for applications such as turbine bearings in aircraft engines and high-temperature mold release. Adding HBN to lubricating grease significantly enhances high-temperature lubrication performance.
Balance of insulation and thermal conductivity: HBN has a thermal conductivity of up to 30 W/mK along the c-axis, combined with an ultra-high resistivity (10^16 Ω·cm) and low dielectric constant (ε≈4). This "both insulating and thermally conductive" property makes it an ideal filler material for high-performance electronic packaging materials. Incorporating HBN nanosheets into polymer matrices (such as epoxy resin) can increase thermal conductivity by 3-5 times without significantly increasing electrical conductivity.
Neutron absorption protection: Boron-10 isotopes have a capture cross-section of up to 3,840 target eV for thermal neutrons, making HBN an excellent candidate for nuclear reactor control rods and protective materials. Neutron absorption capacity can be further enhanced through isotope enrichment technology.
Two-dimensional material growth substrate: HBN has atomically flat surfaces and no dangling bonds, making it an ideal substrate for preparing high-quality two-dimensional materials (such as graphene and MoS2). The mobility of two-dimensional electronic devices grown on it can be improved by an order of magnitude.
4 CBN: Structure and Applications
4.1 Synthesis
Cubic boron nitride (CBN) adopts a zinc blende crystal structure, making it a fully synthetic material not found in nature. With nearly 100% sp3-bonding, its B-N bond length (1.568 Å) slightly exceeds diamond's C-C bond (1.54 Å). Despite this, the ionic character of B-N bonds contributes to exceptional bond strength, endowing CBN with superhard properties.

Fig. 3 cBN Crystals
CBN synthesis predominantly relies on high-temperature/high-pressure (HTHP) technology under typical conditions:
Pressure: 5–7 GPa (~50,000–70,000 atm)
Temperature: 1400–1800°C
Catalysts: Alkali/alkaline earth compounds (e.g., Mg3BN2, Li3N)
In HTHP processing, hexagonal boron nitride (HBN) mixed with catalysts is sealed in pyrophyllite capsules and compressed in belt-type or multi-anvil presses. Under ultrahigh pressure, molten catalysts facilitate the phase transition from HBN to CBN. The synthesized products are acid-washed to remove catalyst residues, yielding CBN microcrystals or sintered polycrystalline aggregates.
While chemical vapor deposition (CVD) has emerged as a low-pressure alternative for CBN thin films, challenges remain in growth rate, crystal quality, and adhesion strength on foreign substrates—key hurdles for industrial adoption.
4.2 Performance Advantages and Industrial Applications
The zincblende crystal structure of cubic boron nitride (CBN) enables a unique combination of properties through its 100% sp3-bonded network. Each boron (B) and nitrogen (N) atom forms tetrahedral coordination with a bond length of 1.568 Å, slightly longer than diamond's C-C bond (1.54 Å). However, the ionic character of B-N bonds (electronegativity difference ΔEN=1.0) increases bond energy to 4.0 eV, achieving a microhardness of 40-50 GPa, second only to diamond. This atomic architecture underpins three critical advantages:
Thermal Stability
While diamond tools oxidize above 800°C, CBN retains stability up to 1,300°C in air and 1,400°C in inert atmospheres. This resilience stems from:
High bond energy: B-N bonds (389 kJ/mol) exceed C-C bonds (347 kJ/mol) in strength;
Self-passivating oxide layer: Dense B2O3 (melting point 450°C) inhibits oxygen diffusion.
These properties make CBN the only viable superhard material for high-speed dry machining, operating reliably at cutting-zone temperatures exceeding 1,000°C while eliminating coolant costs.
Chemical Inertness Toward Ferrous Metals
Diamond undergoes catastrophic catalytic graphitization when machining iron, nickel, or cobalt. In contrast, CBN's absence of unpaired electrons and low formation enthalpy (-250 kJ/mol) ensures complete inertness. This addresses a critical gap in ultra-precision manufacturing by providing a chemically inert and thermally stable cutting tool for ferrous materials.
Table 2 Chemical Inertness Toward Ferrous Metals and Applications
|
Application |
Performance Advantage |
Industrial Impact |
|
Engine block/crankshaft machining |
50× longer tool life vs. carbide |
Processes 10,000+ gear parts per insert |
|
Inconel 718 machining |
Cutting speeds ≥500 m/min |
300% efficiency gain, 40% cost reduction |
|
High-chrome cast iron roll |
Surface roughness Ra<0.8 μm |
Eliminates thermal damage from grinding |
5 WBN: Structure and Applications
5.1 Bridging Structure and Functionality
Wurtzite boron nitride (WBN) occupies a unique metastable position within the BN phase diagram, combining hexagonal symmetry (P6₃mc space group) with full sp3 tetrahedral bonding. Unlike its layered cousin, h-BN, WBN adopts an ABAB stacking sequence along the c axis, structurally analogous to AlN, which induces intrinsic polarization. This configuration enables theoretical piezoelectric coefficients (d33≈5–8 pC/N), positioning WBN as a candidate for ultrahigh-temperature sensors.
Synthesis Challenges and Pathways
Producing phase-pure WBN demands extreme conditions or kinetic control:
Shock wave synthesis (10–50 GPa, μs-duration) delivers gram-scale output but suffers from high dislocation densities (>10^12 cm^-2), limiting functional applications.
Catalytic high-pressure methods (5–8 GPa, 1500–2000°C) using MgB2 catalysts yield superior crystallinity through direct h-BN→WBN transformation.
Plasma-enhanced CVD emerges as a scalable thin-film route: By tuning ion bombardment energy and substrate bias on Si(111) at <800°C, oriented growth is achievable, though deposition rates remain below 2 μm/hour.
Table 3 WBN's Anisotropic Structure Manifests in Distinctive Properties
|
Structural Driver |
Measured Outcome |
|
sp^3 bonding density |
Hardness: 30 GPa (vs. 40–50 GPa for CBN) |
|
Polar *c*-axis |
Bandgap: 5.8 eV (deep-UV transparency) |
|
Stacking anisotropy |
Thermal conductivity: 15 W/m·K (in-plane) / 8 W/m·K (cross-plane) |
Overcoming Metastability Barriers
The Achilles' heel of WBN lies in its thermodynamic instability:
Above 1700°C at ambient pressure, it reverts to h-BN.
Epitaxial stabilization via AlN/GaN buffer layers suppresses phase degradation, while pulsed laser annealing reduces stacking fault density by 60% (per Advanced Materials 35, 2209143).
First-principles calculations suggest BeO alloying could enhance piezoelectric response by 40%, though experimental validation is pending.
Emerging Application Frontiers
WBN's property profile unlocks domains inaccessible to conventional materials:
1. >1000°C piezoelectrics: Outperforms PZT ceramics in turbine condition monitoring.
2. Deep-UV photonics: Enables sub-220 nm optoelectronic devices for sterilization and lithography.
3. Thermal management: Anisotropic heat spreading in GaN HEMTs reduces hot-spot temperatures by 18%.
Fig. 4 Deep-UV
5.2 Properties and Applications
Despite its current laboratory-stage development, Wurtzite Boron Nitride (WBN) exhibits compelling characteristics with disruptive potential:
Mechanical Performance Beyond Conventional Materials
Theoretical models predict WBN hardness exceeding 40 GPa, approaching cubic BN (CBN) levels. Experimental nanoindentation confirms 35–38 GPa hardness, surpassing tungsten carbide (15–20 GPa) but slightly below CBN (40–50 GPa). Significant anisotropy exists, with peak hardness on the (001) crystallographic plane. This positions WBN as a candidate for specialty cutting tools in abrasive environments.
Electronic Structure Advantages
First-principles calculations suggest WBN may possess a direct bandgap near 5.8 eV, contrasting with the indirect gaps of h-BN (5.9 eV) and CBN (6.4 eV). If experimentally verified, this would enable:
Deep-UV optoelectronics: Efficient emitters/detectors below 220 nm wavelength for sterilization and lithography
High-energy photon sensing: Solar-blind detectors with 30% higher quantum efficiency than AlGaN
Power Electronics Potential
WBN's combination of low permittivity (ε ≈ 4.5) and high breakdown field (>10 MV/cm) creates opportunities in extreme-condition electronics:
Table 4 Comparison of Power Electronics Potential
|
Property |
WBN Value |
Benchmark Comparison |
|
Baliga Figure of Merit |
~3× SiC |
Enables 60% smaller power devices |
|
Thermal Stability |
>1000°C |
2× GaN operational limit |
|
Neutron Cross Section |
760 barns |
40% lower than SiC (nuclear apps) |
Extreme Environment Resilience
Superior oxidation resistance of h-BN compared to w-BN at temperatures exceeding 1200°C, coupled with a high neutron capture cross-section (~760 barns), suggests applications in:
-
Nuclear reactor sensors: In-core flux monitors surviving 10^21 n/cm^2 fluence
-
Downhole electronics: Drilling telemetry systems operating at 300°C/15 kpsi
-
Plasma-facing components: Divertor coatings in fusion reactors
6. Comparative Analysis and Future Trajectories
The ternary system of hexagonal (h-BN), cubic (c-BN), and wurtzite (w-BN) boron nitride exhibits complementary properties that define their technological niches. A multidimensional performance matrix reveals critical trade-offs:
6.1 Property Benchmarking
Mechanical Performance
c-BN dominates ultrahard applications with 40–50 GPa hardness and wear resistance 50× superior to carbide tools
h-BN excels as a solid lubricant (coefficient of friction 0.15) and a machinable ceramic
w-BN demonstrates balanced toughness (K1c≈4 MPa·m^0.5) at 35–38 GPa hardness
Table 5 Comparative Analysis of Boron Nitride Polymorphs: Thermal, Electronic, and Economic Profiles
|
Property |
h-BN |
c-BN |
w-BN |
|
Thermal Management |
|||
|
Thermal conductivity |
20–30 (in-plane) |
13–20 (isotropic) W/m·K |
12–18 (predicted) |
|
Thermal expansion |
-0.4×10^-6/K (in-plane) |
2.7×10^-6/K |
3.1×10^-6/K (a-axis) |
|
Electronic Properties |
|||
|
Bandgap type |
Indirect (5.9 eV) |
Indirect (6.4 eV) |
Direct (5.8 eV) |
|
Dielectric constant |
ε∥= 5.1 |
4.5 |
4.8 |
|
Breakdown field |
5–7 MV/cm |
>10 MV/cm |
>8 MV/cm |
|
Baliga FOM |
N/A |
3× SiC |
5× SiC |
|
Economic Viability |
|||
|
Production scale |
Industrial (>10k tons/yr) |
Niche (PCBN tools) |
Lab-scale only |
|
Cost |
<$100/kg |
$200–500/kg (grit) |
>$5,000/kg |
|
Key commercial form |
Lubricants/Cosmetics |
Cutting tools |
No commercial product |
6.2 Industrial Landscape and Technical Hurdles
The industrialization maturity of boron nitride polymorphs diverges significantly. Hexagonal BN (h-BN) dominates global production with over 10,000 metric tons of annual output, primarily serving lubricant and cosmetic markets at costs below $100/kg. Yet its advancement is constrained by limited single-crystal growth capabilities beyond 50 mm and persistent stacking faults in large-area films.
Cubic BN (c-BN) occupies a high-value niche through polycrystalline tools (PCBN), driving a $1.5 billion market (2023) with 8-10% annual growth. While abrasives cost $200-500/kg and cutting inserts $50-200/piece, two critical bottlenecks persist: the inability to synthesize single crystals larger than 3 mm, restricting high-precision optics applications, and sluggish CVD deposition rates under 5 μm/hour that hinder thin-film adoption.
Wurtzite BN (w-BN) remains firmly in the laboratory domain, with synthesis costs exceeding $5,000/kg and fewer than 50 peer-reviewed studies published annually. Its path to commercialization hinges on solving dual challenges: establishing reproducible bulk synthesis protocols and experimentally confirming the predicted direct bandgap, a prerequisite for optoelectronic applications.
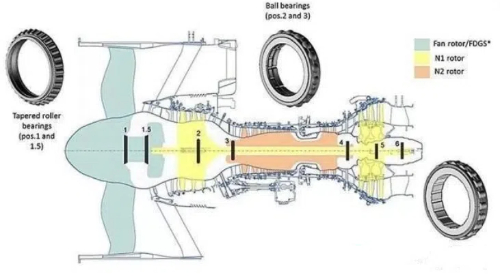
Fig. 5 Aviation Bearing Structure
6.3 Emerging Frontiers and Convergent Innovation
Future breakthroughs will emerge from cross-cutting strategies that exploit the synergistic properties of BN polymorphs:
Atomic-Level Design
Defect engineering transforms limitations into opportunities: nitrogen vacancies in c-BN demonstrate 1.8 ms coherence times at 300K – rivaling diamond NV centers for quantum sensing – while boron vacancies in h-BN enable room-temperature single-photon emission at 580 nm for secure communications. Concurrently, heterostructure integration combines material strengths, such as aerospace bearings with c-BN wear surfaces (10 μm), w-BN transition layers (5 μm), and h-BN solid-lubricant bases (20 μm). This hierarchical design tripled service life in JAXA turbine tests compared to tungsten carbide.
Dimensional Control
Reducing dimensionality unlocks quantum phenomena:
BN nanotubes (BNNTs) achieve 30 GPa tensile strength while maintaining 5.7 eV bandgaps, enabling radiation-hard composites for satellite structures
c-BN quantum dots exhibit size-tunable emission from 230-400 nm, creating pathways for deep-UV biosensors
w-BN nanowires theoretically generate 85 mV·m/N piezoelectric coefficients for self-powered microsystems
Table 6 Extreme Environment Deployment
|
Application |
BN Material |
Performance Threshold |
|
Deep-earth probes |
c-BN |
10 km depth, 400°C, 150 MPa |
|
Fusion reactor liners |
w-BN |
>100 dpa neutron irradiation |
|
Venus surface electronics |
h-BN |
470°C in corrosive atmosphere |
7 Conclusion
The remarkable divergence in properties exhibited by hexagonal, cubic, and wurtzite boron nitride—from h-BN's graphite-like lubricity to c-BN's diamond-rivaling hardness and w-BN's predicted direct bandgap—serves as a textbook demonstration of how atomic-scale architecture dictates macroscopic performance. This polymorphic spectrum, governed by the transition from sp² to sp³ hybridization and crystalline symmetry variations, enables tailored solutions across engineering frontiers. Industrial maturation follows distinct trajectories: h-BN dominates thermal management markets with 10,000-ton annual production, while c-BN's $1.5 billion tool industry grows at 8% CAGR through superhard machining applications. WBN remains at a pivotal threshold, where experimental validation of its 5.8 eV direct bandgap could unlock deep-UV optoelectronics if synthesis costs breach the $500/kg barrier.
Convergent innovation now blurs traditional material boundaries. Heterostructures marrying c-BN's wear resistance with h-BN's lubricity and w-BN's toughness triple component lifetimes in aerospace extreme environments. Quantum technologies leverage atomic-scale defects—nitrogen vacancies in c-BN achieve 1.8 ms coherence times at 300K, while h-BN's boron vacancies emit single photons at 580 nm—creating pathways for room-temperature quantum devices. Beyond terrestrial limits, BN materials enable technologies operating where conventional systems fail: h-BN withstands Venus' corrosive 470°C atmosphere, w-BN tolerates >100 dpa neutron flux in fusion reactors, and c-BN tools probe Earth's crust beyond 10 km depths. As synthesis science advances to harness these polymorphic synergies, boron nitride continues to redefine the art of the possible in materials engineering.
Stanford Advanced Materials (SAM) offers a range of high-quality boron nitride products, including hexagonal boron nitride (h-BN), pyrolytic boron nitride (PBN), and custom machined BN parts.

 القضبان
القضبان
 الخرز والكرات
الخرز والكرات
 البراغي والصواميل
البراغي والصواميل
 البوتقات
البوتقات
 الأقراص
الأقراص
 الألياف والأقمشة
الألياف والأقمشة
 الأفلام
الأفلام
 فليك
فليك
 الرغاوي
الرغاوي
 رقائق معدنية
رقائق معدنية
 الحبيبات
الحبيبات
 أقراص العسل
أقراص العسل
 الحبر
الحبر
 صفائح
صفائح
 الكتل
الكتل
 التشابك
التشابك
 غشاء معدني
غشاء معدني
 اللوحة
اللوحة
 المساحيق
المساحيق
 قضيب
قضيب
 الصفائح
الصفائح
 البلورات المفردة
البلورات المفردة
 هدف الاخرق
هدف الاخرق
 الأنابيب
الأنابيب
 الغسالة
الغسالة
 الأسلاك
الأسلاك
 المحولات والآلات الحاسبة
المحولات والآلات الحاسبة
 اكتب لنا
اكتب لنا




 Chin Trento
Chin Trento

